Ion Exchange can be used in wastewater treatment plants to swap one ion for another for the purpose of demineralization. There are basically two types of ion exchange systems, one which is using the anion resins and another is the cation exchange resins. The materials can be further broken down to individual grouping depending on whether it is a strong base cation, weak acid cation, strong base anion, or weak base anion. Regardless of which type of exchangers, the resins can very sensitive to fouling caused by presence of organic matter in the untreated water. Thus it is imperative that before the influent undergoes the treatment process, another separate section is needed to remove most of the suspended solids and if possible the soluble organics as well so that it will put lesser loads towards the ion exchange unit.
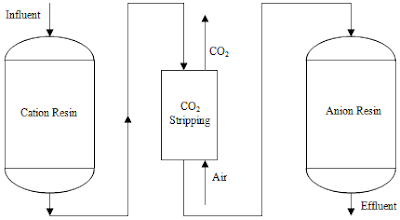 A typical scenario on how the whole process works can be simplified in the example given here. For instance, in a common demineralizer, influent water which passes through a cation exchange resin will be stripped off of it metallic cations salt to become acids whereby loss of the ions will be replaced with a similar corresponding amount of hydrogen ions. The resultant acids will then be removed through another alkaline regenerated anion exchange resins in which this time round, the anions present in the wastewater will be substituted with equivalent amount of hydroxides. As the capacity of the bed always has a certain fixed limit, eventually the resin will become exhausted and thus has to undergo regeneration process. The cation exchange resin is regenerated using either hydrochloric or sulfuric acid, producing waste brine in the process while the anion resin will be regenerated with sodium hydroxide.
A typical scenario on how the whole process works can be simplified in the example given here. For instance, in a common demineralizer, influent water which passes through a cation exchange resin will be stripped off of it metallic cations salt to become acids whereby loss of the ions will be replaced with a similar corresponding amount of hydrogen ions. The resultant acids will then be removed through another alkaline regenerated anion exchange resins in which this time round, the anions present in the wastewater will be substituted with equivalent amount of hydroxides. As the capacity of the bed always has a certain fixed limit, eventually the resin will become exhausted and thus has to undergo regeneration process. The cation exchange resin is regenerated using either hydrochloric or sulfuric acid, producing waste brine in the process while the anion resin will be regenerated with sodium hydroxide.
Individual ion exchange bed, other than grouped together according to its functional groups can also be divided into different types based on its solubility. Naturally for it to be effective, the materials must be insoluble under normal conditions and for that to happen, usually high molecular weight compound has to be selected. Bead size is also another criteria that determines the type of resin suitable for general application. Basically a certain granular size is needed so that the molecules that form the whole structure do not hold on together too compact and there must be a certain void volume to prevent massive liquid head loss. The characteristics on how the beads are arranged will also affect the ion exchange bed physical durability whether it is good enough to withstand expansion and contraction due to change of temperature without risk of bursting or collapsing taking place. One more thing to take note is that depending on the procedures used for backwashing and especially in cases whereby there is an uncontrolled high flowrate caused by mishandling, this can lead to blowoff of resins coming out from the vessel and resulting in capacity loss of the beds. Therefore, typical attrition losses can be expected to be within 5 to 20% depending on which type of ion exchange resin used in the systems.
Ion exchange softening, also known as Sodium Zeolite Softening is a typical example of how the ion exchange process works to purify the water by removing the hardness level caused by presence of calcium and magnesium. This is normally used in steam boilers and industrial water treatment applications. When the bed gets completely saturated with Ca and Mg ions, it has to be regenerated mainly by increasing the amount of sodium ions. This is done by first performing backwashing to minimize compactness of the bed and to release trapped air pockets. After that, it will be followed by the actual regeneration process using brine solution. Once this is completed, rinsing will be carried out to remove whatever excess brine left in the exchange bed. In modern designs, all these operations are performed using automated programs which can detect the level of saturation and prompt the operators to act accordingly. Overall, depending on the influent characteristics, this will determine the frequency needed to carry out the whole cycle of regeneration.
Related water and wastewater treatment technology operating on similar working principles: Electrocoagulation


